New Model and Gene Targeting Approach Show Promise for ALS Therapies
Researchers from the Project ALS Therapeutics Core at Columbia (the Core), in a groundbreaking collaborative effort with Ionis Pharmaceuticals, have developed a new tool for studying ALS mechanisms and therapies, as well as a novel approach for treating ALS caused by mutations in the fused in sarcoma (FUS) gene. These developments, led by Vladislav Korobeynikov and others from Neil Shneider’s lab at Columbia University, were published in Nature Medicine last month, highlighting a major step toward improving the development of reliable therapeutics for people living with ALS and showing promise for early and preventative care in those who might develop FUS-ALS in the future.
Why focus on FUS?
Mutations in the FUS gene cause an extremely aggressive, early onset form of ALS. While this particular form of ALS is not common among people with the disease, its relatively quick progression makes FUS-based ALS valuable for studying disease mechanisms and symptom progression in the lab, and for rapidly evaluating how effective different therapies are in animal models.
Seeking to harness the aggressiveness of FUS-ALS and hoping to improve on current ALS models, Korobeynikov developed a novel mouse model with a FUS mutation. Previously, animal models of ALS were developed via genetic engineering, whereby mutated genes associated with ALS were inserted in unnaturally high quantities into random locations in the mouse genome. While this method has been able to replicate some facets of the disease that are experienced by people living with ALS, it has not been a consistently reliable model for understanding many forms of ALS and testing potential ALS therapies. In this new paper from the Shneider lab and other researchers at the Core, Korobeynikov and colleagues used a more nuanced approach to better replicate ALS as it appears in people. Specifically, they replaced the healthy FUS gene in the mouse genome with a mutated version of the FUS gene that’s been shown to cause ALS. This induced a strong, rapid symptom progression that closely matched ALS in people, including loss of motor neurons and their connections to muscles, as well as neuroinflammation–an immune response in the nervous system that can worsen ALS disease progression over time.
A New Treatment for FUS-ALS
To target this cellular mechanism that causes FUS-ALS, researchers at the Core tested ION363 in their new FUS-ALS mice. This drug is an antisense oligonucleotide (ASO), an emerging form of precision medicine that specifically finds mutated genes and prevents them from being converted into proteins that, in this case, cause neurodegeneration (or loss of motor neurons). Korobeynikov and colleagues found that administering ION363 did, indeed, improve symptoms in the new FUS-ALS mouse model.
Thanks to the collaborative efforts of the Core, the Motor Neuron Center and the Eleanor & Lou Gherig ALS Center at Columbia, and Ionis Pharmaceuticals, ION363 was then administered to a young woman with FUS-ALS: Jaci Hermstad, for whom ION363 received its alternate name: jacifusen. Jaci’s courage to try jacifusen yielded promising discoveries: jacifusen was not only safe for Jaci in multiple dosages; it also successfully reached her neurons—a difficult feat for many drugs against neurodegenerative processes.
In the image below, you can see FUS protein accumulation (red clumps) in the spinal cord of a person with FUS-ALS (middle panel). Following multiple doses of ION363, FUS protein accumulation is no longer visible in the spinal cord of a person with FUS-ALS (right panel), more closely resembling the spinal cord sample of a healthy individual (left panel). This demonstrates that ION363 successfully targets the nervous system, allowing it to improve motor neuron health.
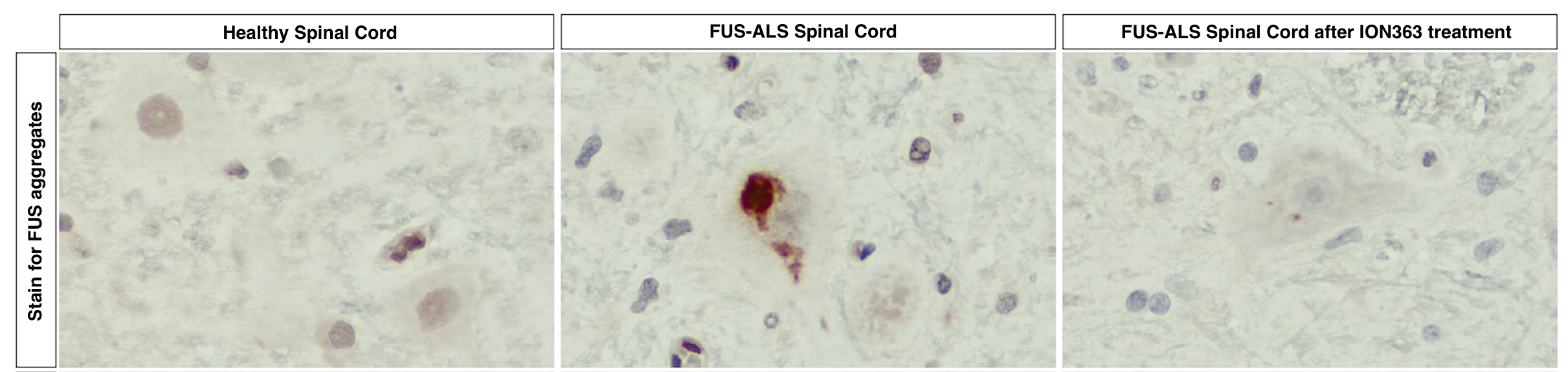
Adapted from Korobeynikov et al., 2022 – Figure 5e
Below, you can also see that Jaci’s ALS Functional Rating Score-Revised (ALSFRS-R), a scale for measuring motor function and disease progression in people living with ALS, decreased rapidly, then held steady during ION363 treatment. This suggests that ION363 effectively slowed Jaci’s functional decline.
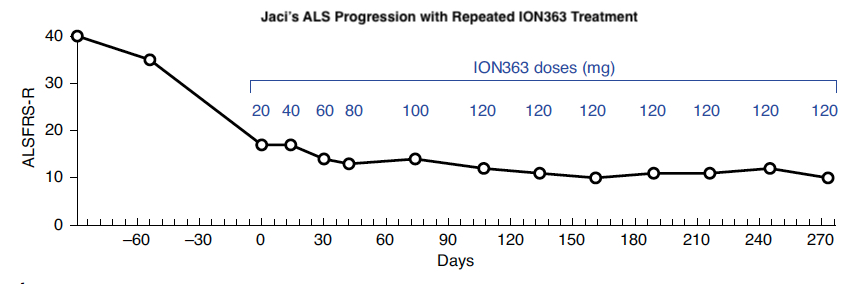
Adapted from Korobeynikov et al., 2022 – Figure 5a
Next Steps at the Core
Use in only one person with FUS-ALS does not allow us to make conclusions about how effective jacifusen is in treating ALS; however, the Core and collaborators are now further testing its safety and effectiveness in FUS-ALS patients through a formal, FDA-approved clinical trial by Ionis Pharmaceuticals. Further, researchers at the Core are already using the new FUS-ALS mouse generated in this study to test other therapeutic approaches that target common cellular mechanisms of ALS, and could, therefore, work across a broader range of ALS types. Finally, recent speculations about ASO function in neurodegeneration, specifically in ALS, posit that this type of drug might be most effective early in disease progression or even as a preventative measure before diagnosis. As researchers at the Core establish which lipid-based biomarkers indicate presymptomatic ALS, efforts to deliver ASOs to people showing early biomarkers of ALS before symptom onset could improve their outcomes.
Broader Impacts for ALS and Other Neurodegenerative Diseases
Every cell in the body is constantly translating genes (DNA) into intermediate molecules (RNA) before making them into the machines and structures that dictate basic cellular health and function (proteins). RNA-binding proteins, like FUS, ensure that this process of converting genetic information into cellular machinery goes smoothly. Mutations in RNA-binding proteins disrupt this conversion process, resulting in protein accumulation in cells, a common factor across different types of ALS and other neurodegenerative diseases, like Alzheimer’s Disease. By developing both a FUS-ALS animal model and therapies to treat FUS-ALS, the Core is improving our ability to understand and treat multiple types of ALS, and could even impact therapy development for other neurodegenerative diseases.
These developments come almost three years to the day since Jaci Hermstad was diagnosed with FUS-ALS. To learn more about Jaci and the Hermstad family’s journey with Project ALS, check out A Cowgirl’s Courage: The Jaci Hermstad Story. Jaci’s courage and sacrifice for the development of effective ALS therapies will always be remembered.
Korobeynikov, V.A., Lyashchenko, A.K., Blanco-Redondo, B. et al. Antisense oligonucleotide silencing of FUS expression as a therapeutic approach in amyotrophic lateral sclerosis. Nat Med 28, 104–116 (2022). https://doi.org/10.1038/s41591-021-01615-z



