Last year, Project ALS launched The Project ALS Therapeutics Core at Columbia (THE CORE), a 3-year, $6.3M initiative toward the first meaningful therapies for ALS. Led by scientific co-directors Hynek Wichterle, PhD, Neil Shneider, MD, PhD, and Serge Przedborski, MD, PhD, THE CORE is the world’s first and only partnership between a world-class academic institution and a non-profit organization dedicated to a full-spectrum approach to ALS drug screening, pre-clinical evaluation, and improved human clinical trials.
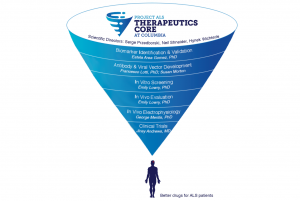
In its first year, THE CORE has tested thousands of chemical compounds and FDA-approved drugs in cellular models of ALS, established drug screening and optimization collaborations with other academic institutions and biotech companies, and developed new cutting-edge tools toward better ALS therapeutic discovery and development. But in recent months, COVID-19 has profoundly affected the day-to-day operations of Project ALS and THE CORE. On March 15th, Columbia University stopped most on-site, in-person research in response to the pandemic. Nevertheless, we understand that ALS cannot–and does not–wait, and through regular remote meetings and innovative new approaches to lab work, THE CORE has continued to push research forward in this uncertain time. A few highlights:
- Prosetin progress. We have kept you updated on our investigational new drug candidate, Prosetin, and our progress toward IND submission with our external partners Charles River Laboratories and Patheon by Thermo Fisher Scientific. In parallel, our team at THE CORE, including Emily Lowry, PhD and Jinsy Andrews, MD, MSc, have identified a potential clinical biomarker for Prosetin, developed a Phase 1 clinical trial protocol, and kept additional efficacy studies in pre-clinical ALS models moving forward.
- Next-generation drug development. A collaboration between THE CORE and medicinal chemists Brent Stockwell (Columbia), Arie Zask (Columbia), and Kent Kirschenbaum (NYU), has yielded several improved versions of chemical compounds called paullones. Paullones are best-in-class motor neuron protectors in lab models of ALS, but cannot be used in people because they cannot get into the bloodstream or brain. Now, THE CORE will test these optimized paullone compounds to see which one works best—and if it checks all our boxes, move it forward to our in vivo drug evaluation unit.
- New techniques to improve pre-clinical testing. A major problem in ALS drug development—and a big reason we started THE CORE—is that a drug’s success in pre-clinical evaluation has not translated to that drug actually helping people with ALS. THE CORE’s electrophysiology unit, led by George Mentis, PhD, is developing more sensitive ways to measure a potential ALS drug’s impact on motor neurons, the cells impacted in ALS. During these past months, our team analyzed data from the past year’s work, and consulted with experts including Eiman Azim, PhD (Salk Institute) to refine our battery of pre-clinical drug tests even further.
Researchers at THE CORE returned to the lab this week with a long list of exciting experiments to get to work on—stay tuned for updates from each Unit Director of THE CORE, and from the external collaborators we’re working with toward better therapeutic options in ALS.



