Motor neurons project out to the muscles in our limbs to drive muscle contraction, allowing us to speak, breathe, and move. These cells are housed in the spinal cord and brain. When someone has ALS, their motor neurons become unhealthy and die, making these cells the primary target for treating ALS. However, because motor neurons are located in the spinal cord and brain, they are difficult to apply therapies to directly—both because their physical location is hard to safely access, and because the blood brain barrier, a structure between the central nervous system and the rest of the body that prevents toxins from entering the nervous system, makes therapeutic delivery to neurons very difficult. A key goal of ALS research has been to find the most effective ways to deliver potential therapies to motor neurons. In 2003, thanks to ground-breaking research supported by Project ALS, scientists harnessed the power of viruses to effectively reach motor neurons with potential ALS therapies.
Hijacking Viruses to Deliver Healthy Molecules
Viruses are the tiny microbes that cause a number of illnesses, ranging from the flu to rabies to COVID-19. Because they are not living organisms with their own cellular machinery, viruses survive and thrive by efficiently invading the cells of host organisms (like us), and injecting their genetic material into our cells. Our cells then replicate their genes for them, allowing viruses to spread to other cells in our bodies and to other hosts. Different types of viruses have different ways of spreading from cell to cell, and different affinities for being spread through specific types of tissues in our bodies. These specific attributes of viruses are controlled by how their outer shells interact with proteins on the outside of our bodies’ cells, and how their genetic materials interact with the molecular machinery in our cells that replicate genes. All of this explains why some viruses primarily target respiratory tissues, like the flu targets our lungs, and others target the nervous system, like rabies targets the brain. Scientists have learned how to turn the tables on viruses, cleverly hijacking their invasive capabilities to efficiently deliver helpful genes to our cells suffering from disease.
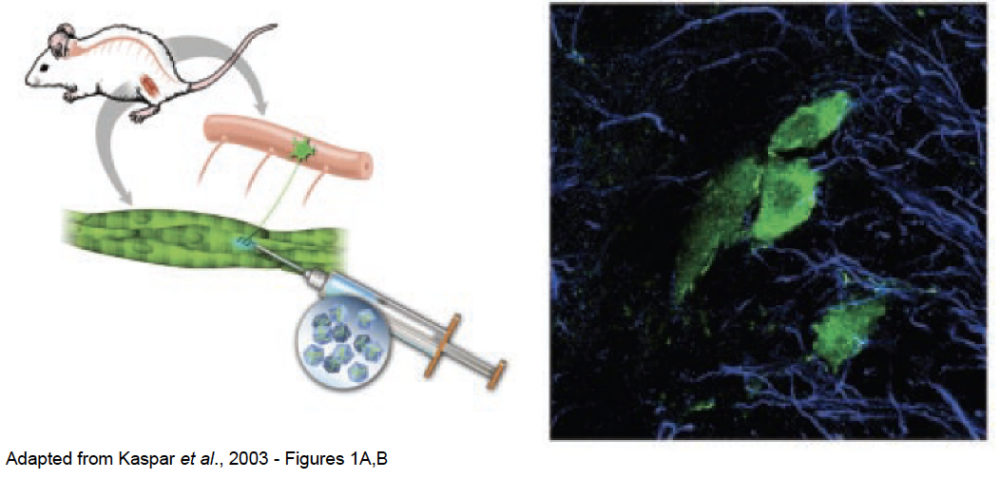
Paving the way specifically for gene therapy methods in neuromuscular diseases like ALS in 2003, Brian Kaspar and colleagues discovered that injecting a particular type of virus into muscles allows the delivery of genes and molecules into motor neurons in the spinal cord. Essentially, Kaspar engineered a specific type of viral vector—the outer shell of a virus that allows it to transfer from cell to cell in its host’s body and share its genetic materials with each cell it enters—to hold a green fluorescent marker gene and injected it into a mouse’s leg muscles. This resulted in successful transfer of this gene from the leg muscle into the mouse’s motor neurons in the spinal cord, where the gene was successfully expressed, turning the motor neurons green (shown in the image below).
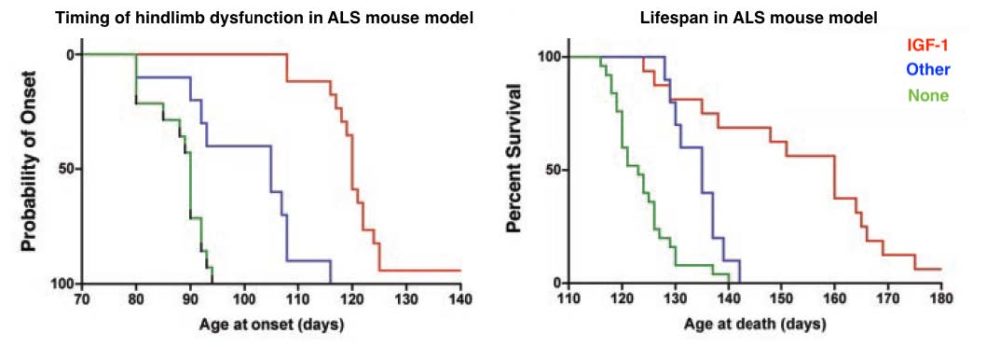
Kaspar and colleagues then used this viral technique to deliver protective molecules called neurotrophic factors to motor neurons in an ALS mouse model. As seen in the graphs below, delivery of the neurotrophic factor IGF-1 (shown in red) in particular effectively slowed neurodegeneration and ALS progression in the ALS mouse model compared to delivery of other neurotrophic factors (shown in blue) or no treatment (green). This work was the first demonstration that viral vectors could be used to transport specific genes from limb muscles into motor neurons, an important step in improving the implementation of therapies for ALS.
How Do Viruses Help Us Fight ALS Today?
While later studies demonstrated that IGF-1 might not be effective at protecting motor neurons in people with ALS, as viral delivery technology improves for medical applications, this and other similar protective molecules could be tested as treatments for ALS. Further, these researchers went on to directly decrease the presence of an ALS-linked gene in the motor neurons of an ALS mouse model using similar methods. Ongoing work in the Viral Unit of the Project ALS Therapeutics Core, led by expert Francesco Lotti, has expanded on these initial developments. His team, in a collaborative effort with Serge Przedborski at the Core, have developed further viral tools for testing exactly which genes cause motor neuron degeneration. Now, researchers in the Viral Unit of the Core are working to improve virally-delivered gene therapies in aging motor neurons, and are even developing clever viral and genetic techniques for reprogramming ALS-susceptible motor neurons into motor neurons that have been shown to be more resilient to disease.



